Research Projects
Structural studies of amyloid in disease
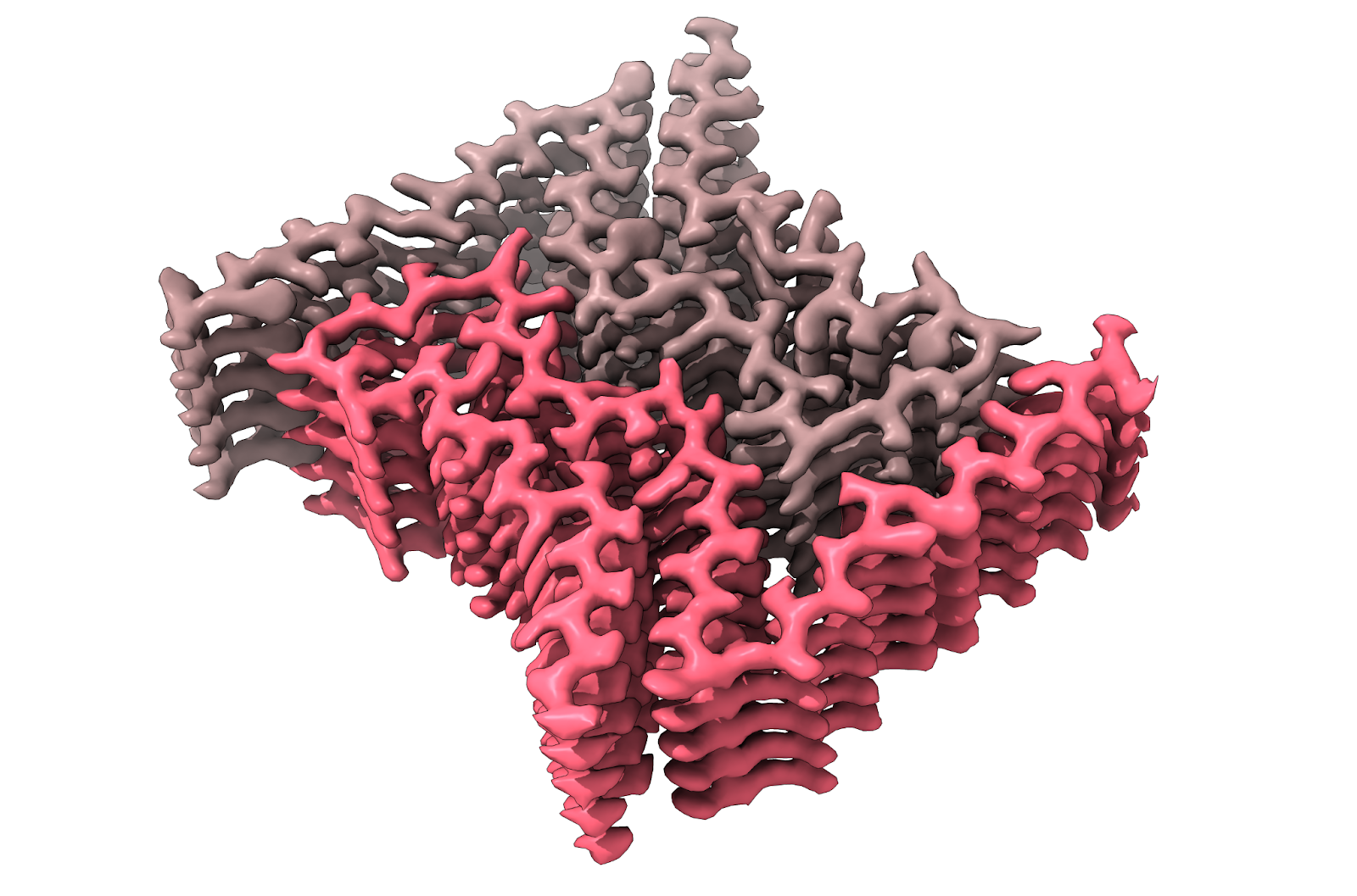
We use many techniques to study the structure and mechanism of amyloid folding and deposition in disease. One of the primary methods we use to study amyloid structures is cryo-electron microscopy (cryo-EM). By determining the structure of amyloid from patient tissues, we can learn about disease-specific mechanisms that cause disease. We use additional biophysical and biochemical techniques to study the propagation, or “seeding” of new amyloid fibrils, as well as the roles short sequences play in aggregation. Altogether, we explore the process of amyloid fibril formation and how they cause disease to better understand how to fight disease in patients.
Design of recognition tools for use in the clinic
Using amyloid structures generated by the field, we are using rational and computational design to create inhibitory agents or detectors for these amyloid fibrils. Our recent focus has been on creating structure-specific probes that can bind to aggregates. These probes allow us to better assess the levels of native protein versus aggregated protein in our in vitro and in vivo studies. The tools we generate can help us better understand the pathology of these amyloidosis diseases and potentially help us create better diagnostic tools or therapeutics.
Using specific probes to study disease on a large scale
Using our structure-based probes, we aim to translate this technology to a clinical setting by developing high-throughput screening assays that detect amyloid aggregates in patient samples. By optimizing these techniques, we can incorporate these detectors to routine lab tests and help identify early-stage aggregation in high-risk patient populations.